Abstract
Background Eltrombopag is an orally bioavailable small molecule agonist of the thrombopoietin receptor (TPO-R), stimulating platelet production. Preclinical and early clinical data have shown its anti-leukemic activity in myeloid malignancies in addition to improved platelet counts, making eltrombopag a suitable candidate treatment following induction therapy for acute myeloid leukemia (AML). Preliminary data suggest that eltrombopag-mediated iron chelation could be involved in its anti-tumoral activity rather than apoptosis induction (Roth M, Blood 2012). In a randomized, double blind placebo controlled study conducted in advanced MDS and AML patients (pts) in relapse, refractory or ineligible to receive standard treatments, eltrombopag yielded a trend for improved overall survival (Frey N, ASH, 2012).
Methods EPAG 2015 was designed as a randomized phase II multicenter trial aiming at assessing the impact on outcome of eltrombopag administered to elderly AML pts receiving induction chemotherapy (NCT 03603795). Inclusion criteria were: ≥ 60 years of age, newly diagnosed AML (de novo or therapy-related) except AML3 (APL) and AML7, favorable or intermediate cytogenetic risk (MRC 2010 classification), ECOG performance status < 3; HCT-CI score < 3, adequate baseline organ function. Exclusion criteria were: AML with adverse cytogenetic risk, or arising from MDS, MPN/CMML, or with BCR-ABL1, known active central nervous system leukemia, history of thromboembolic event or ongoing use of anticoagulation. The induction chemotherapy regimen consisted of one cycle of daunorubicin 60 mg/m²/day (d), d1 to d3, cytarabine 100 mg/m²/d, CIV d1 to d7, lomustine 200 mg/m², orally d1. Eltrombopag 200 mg orally (100 mg/day for pts with East Asian heritage) or placebo (blinded) was given once daily from day 11 until AML response evaluation or platelet counts > 100 x 109/L (maximum d45). The use of GCS-F, anti-fungal, anti-viral and anti-pneumocystisjiroveci prophylaxis was recommended. Pts who failed to reach complete remission (CR/CRi) after this cycle of induction were off-study whereas CR/CRi patients were planned to receive up to 6 courses of mini-consolidation every 30 to 45 days with daunorubicin 60mg/m², d1, cytarabine 50 mg/m²/12h, subcutaneous, d1 to d5. Pts then received 6 months of maintenance therapy alternating mercaptopurin and methotrexate. The addition of midostaurin in patients with FLT3-ITD or FLT3-TKD mutations was not allowed during induction. Allogeneic stem-cell transplantation (ASCT) was allowed after 2 to 4 cycles in pts with intermediate or adverse risk (European LeukemiaNet 2017).
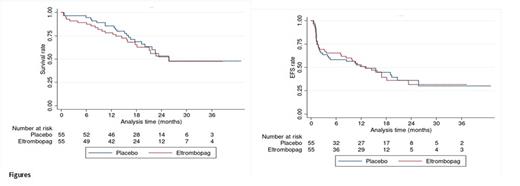
Results From October 2018 to June 2021, 110 pts of a median age of 70 y (range 61-84). were included (55 per arm). There were 71 males (64.5%). Median complete blood count was 2.7x109/L (0.7-142). Pts randomized to receive eltrombopag had significantly less previous histories of cancer (3 vs 12; p = 0.01). Most pts had intermediate risk cytogenetics (77.9%). NPM1 and FLT3 mutations (per local analysis) were observed in 26 (23.6%) and 27 pts (15.4%), respectively. High throughput sequencing of a myeloid gene panel, performed centrally will be presented at the meeting. Following induction, 90 pts (81.8%) achieved CR/CRi (45 in each group), including 74 CR (38 in the eltrombopag group and 36 in the placebo group), while 11 failed (5 and 6) and 6 died early (4 and 2). During induction, a similar rate of grade III-IV adverse events was observed in the two arms (N=29, 52.7% vs 33, 60%, p = 0.4). Severe hemorrhage occurred in 2 pts who received placebo and 1 who received eltrombopag. Central nervous system toxicity was observed only in 2 placebo pts. The number of platelet transfusions was significantly reduced in the eltrombopag group 9.76+ 5.38 vs 12.56 + 9.69, p = 0.063 (10% risk). Of the 90 CR/CRi pts, 85 entered the consolidation phase. Twenty-eight pts received ASCT in first CR, respectively 13 after eltrombopag and 15 after placebo. The median follow-up time for alive pts was 20.9 months. Forty-two pts (77.9%) who received eltrombopag were alive at 12 months vs 46 (85.3%). Event-free survival at 12 months was 52.7% after eltrombopag vs. 50.5%. These differences are not statistically significant.
Conclusion In older AML pts eligible for intensive treatment, adding eltrombopag to induction is feasible and safe, reduces significantly the need of platelet transfusions, yet did not impact survival in this study.
Disclosures
Pigneux:Sanofi: Other: travel; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; takeda: Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizzer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz pharmaceutical: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Dumas:Astellas: Honoraria; Daiichi-Sankyo: Honoraria; Jazz Pharmaceuticals: Honoraria; Abbvie: Honoraria; BMS Celgene: Honoraria; Janssen: Honoraria. Vey:Roche: Honoraria; Novartis: Honoraria, Research Funding; Janssen: Honoraria; Jazz Pharmaceuticals: Honoraria; BMS: Honoraria; Amgen: Honoraria. Hunault:abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; clinigen: Honoraria, Membership on an entity's Board of Directors or advisory committees; jazz pharmaceutical: Honoraria, Membership on an entity's Board of Directors or advisory committees; incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; servier: Honoraria, Membership on an entity's Board of Directors or advisory committees. Carre:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees. Guieze:Abbvie, Beigene, Janssen, Gilead, Roche, AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astrazeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Desbrosses:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Simand:amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; jazz pharmaceutical: Honoraria, Membership on an entity's Board of Directors or advisory committees; astellas: Honoraria. Leguay:clinigen: Honoraria, Membership on an entity's Board of Directors or advisory committees; servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees. Jourdan:Abbvie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Recher:Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, BMS-Celgene, Otsuka, Astellas, Daiichi-Sankyo, Macrogenics, Roche, Takeda, Servier, Pfizer: Other: Advisory role; AbbVie, Amgen, Novartis, BMS-Celgene, Jazz Pharmaceuticals, Agios, MaatPharma, Astellas, Roche, Iqvia, Daiichi-Sankyo: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.